Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approachesMISEV2023Editorial from Corresponding AuthorsEditorial from Editor-in-ChiefAbout the MISEV GuidelinesThe International Society for Extracellular Vesicles (ISEV) first published its “Minimal Information for Studies of Extracellular Vesicles,” in 2014 (MISEV2014) with an updated version published in 2018 (MISEV2018). ISEV are now proud to share the most recent version of the MISEV guidelines, MISEV2023.
The goal of MISEV 2023 is to provide researchers with an updated snapshot of available approaches and their advantages and limitations for production, separation, and characterization of EVs from multiple sources, including cell culture, body fluids, and solid tissues. In addition to presenting the latest state of the art in basic principles of EV research, MISEV 2023 also covers advanced techniques and approaches that are currently expanding the boundaries of the field. MISEV2023 includes new sections on EV release and uptake and a brief discussion of in vivo approaches to study EVs.
Compiling feedback from ISEV expert task forces and more than 1000 researchers worldwide, MISEV 2023 conveys the current state of EV research to facilitate robust scientific discoveries and move the field forward even more rapidly.
What was the process behind MISEV2023?The achievement of publishing MISEV2023 is the culmination of a three-year process, summarised below. You can read more about this process in the concluding section of MISEV2023.
MISEV2023: domainsNew sections in MISEV2023 are highlighted in bold.
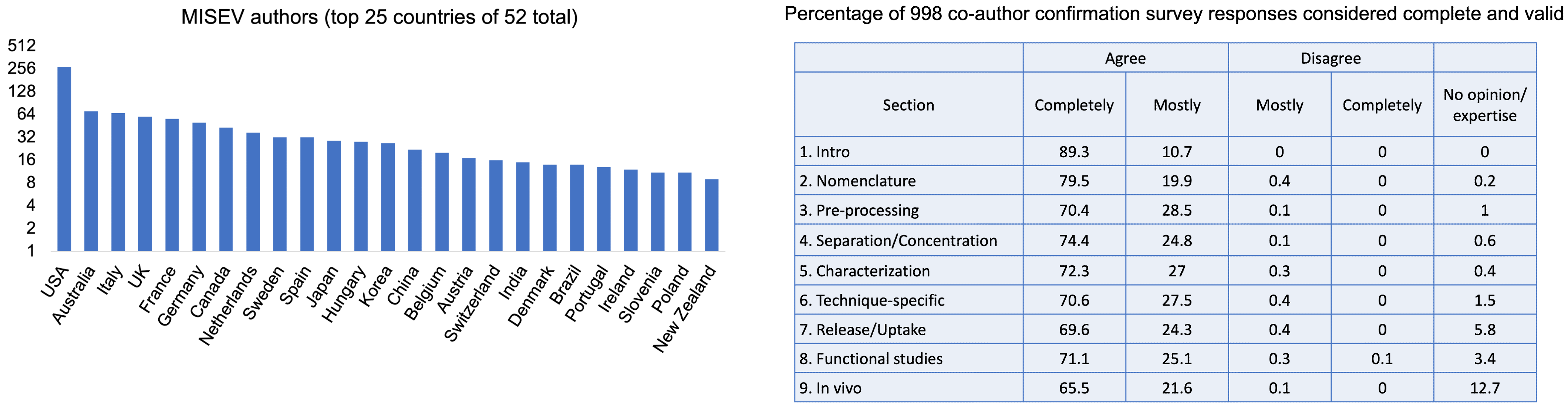
MISEV2023: 1051 co-authors in 53 countries
What MISEV is NOT…or should not be…
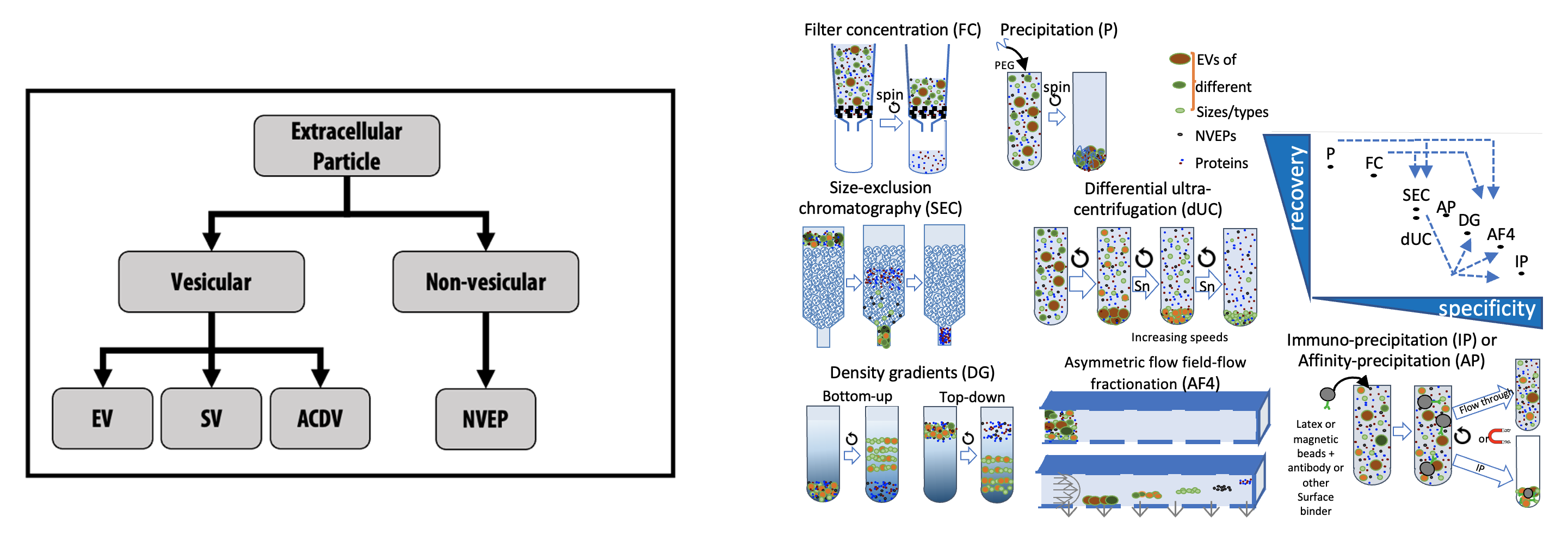
Extracellular particle (EP) nomenclature and separation/concentration methods
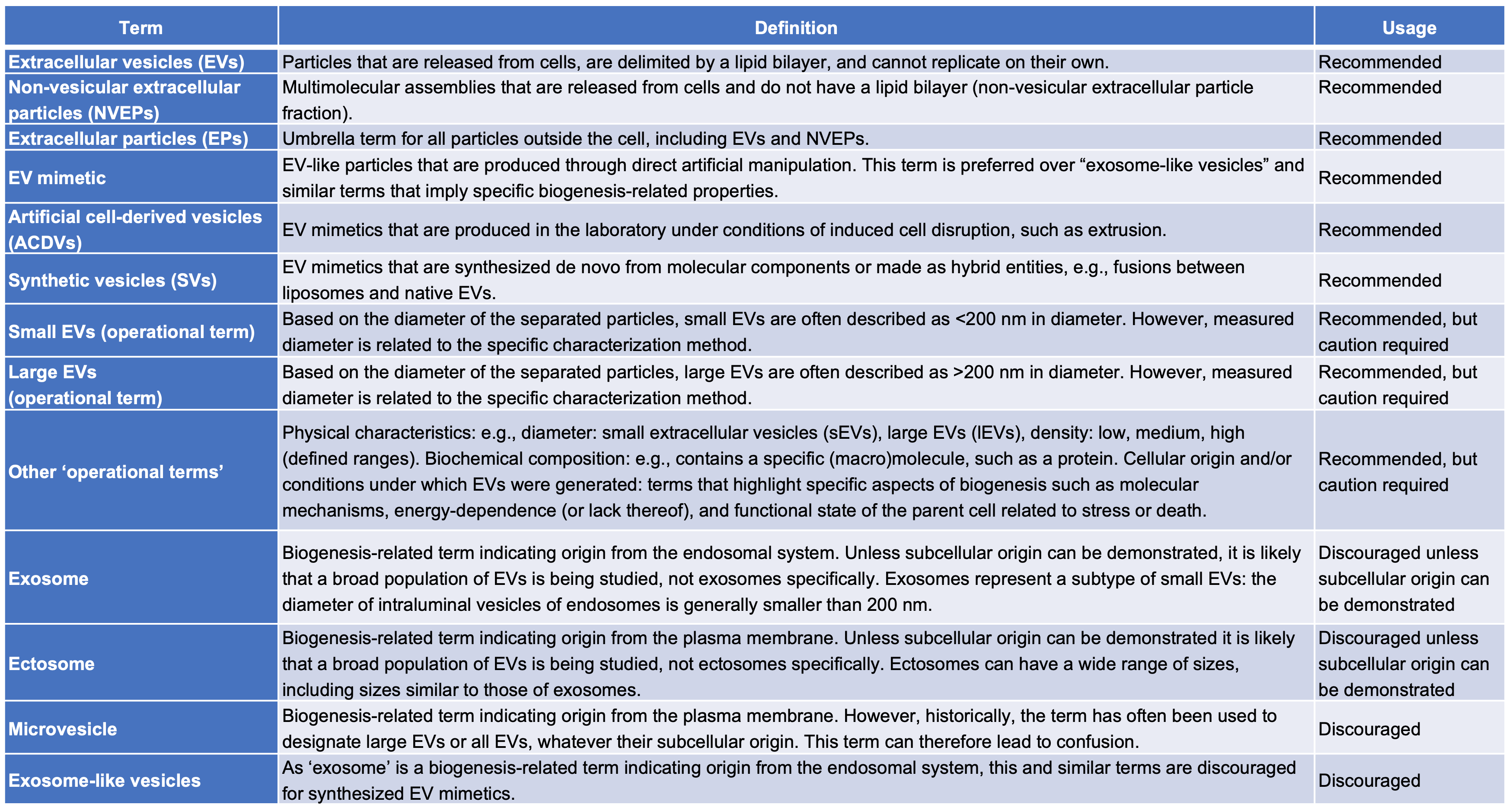
Quick reference on EV terms
Spread the Word!Thanks to community members for featuring MISEV2023. Read more:
Please engage and help us spread the word about this valuable resource.
|